If you vape or are thinking about vaping you’ll probably want to know more about the vaping laws recently imposed by the TPD.
Following a 12-month transition period that went into effect May 20, 2016, new vaping legislation in the form of the “Tobacco Products Directive” (TPD) will be fully implemented and strictly enforced.
Past TPD regulations initially only applied to traditional cigarettes but were expanded in 2014 to include directives aimed at including e-cigs and reclassifying them as tobacco-related products.
However, by May 2016, an abundance of additional TPD laws was newly initiated by the government with the intent of enacting strict regulation upon the vaping industry’s producers, sellers, and importers, as well as the millions of UK citizens who identify as e-cigarette users.
Prompted by an EU directive, the directive immediately impacted not only vapers but also e-cigarette manufacturers and importers but allowed for a special 6-month moratorium for sellers and suppliers to sell old merchandise.
Public outcry led to the formation of a multitude of protest groups, vape-rights activism, and petitions containing thousands of signatures.
Pressured by the collective uproar of thousands of citizens, UK officials arduously struggled with passing TPD directives, with it ultimately passing through Parliament after a turbulent ratification process.
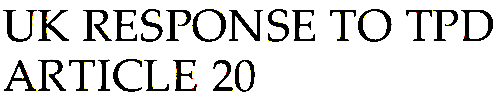

The stringent measures being put into action by the TPD will result in highly controlled e-cigarette and e-liquid sales.
The abundance of measures includes regulations calling for redesigned e-cig packaging constructed to be tamper-proof and child-resistant, in addition to a plethora of newly required labeling information that clearly informs consumers of toxicological and addiction dangers.
Many fo us who currently vape will inevitably experience altered buying practices as we are faced with new tank capacities, maximum volumes, and limited nicotine strengths.
It’s inevitable that some companies will have to put up prices a little.
-Richard Hyslop, IBVTA Chief Executive
While many of us have hotly contested the impending changes that the TPD brings to the United Kingdom, it is also the producers and retailers within the vaping industry vehemently voicing their contention.
New TPD laws will result in extensive control over anyone who engages in the production or sale of e-cigarettes and e-liquids, including sellers on social media, auction sites, or personally-owned websites.
No one is free from the unsparingly exhaustive rules that the TPD is bringing forth.
The new laws have proven to be highly divisive and have prompted the myriad vapers, e-cigarette producers, and sellers populating the UK to continue rising in collective protest against what many refer to as tyrannically oppressive measures.
We have found online sellers selling e-liquids in 100ml bottles with a nicotine strength over 20mg/ml. After May 20 this will be illegal unless the e-liquid is registered as a medicine.
-Alex Fry, Manager at Plymouth Trading Standards
The measures put forth by impending TPD vaping laws are vastly extensive and contain an abundance of complex and esoteric information that only the most up-to-date readers might be privy to.
If you are among the 2.6 million people who use personal vapourizer devices, it is imperative to be clued up on the quickly advancing new rules that will undoubtedly change the way you conduct your vape activities.
We have prepared a comprehensive easy-to-read summary containing all the information you need to fully educate yourself on current and upcoming vaping laws in the UK, the status of TPD, and vape-related fines and punishments for breaking the newly instituted legislation.
TOBACCO PRODUCTS DIRECTIVE HIGHLIGHTS
E-cigarette Tank Capacity:
New TPD regulation:
Disposable e-cigarettes and e-cigarette cartridges and tanks are restricted to containing no more than 2ml of e-liquid.
Impact on vapors:
For heavy users looking to fulfill their cloud-chasing endeavors, the 2ml tank maximum will result in frequent vape refills, which may prove to be frustratingly time consuming and costly.
E-cigarette Contents, Ingredients, and Emissions
New TPD regulation:
Additives like unapproved coloring’s, stimulants such as caffeine and taurine, or anything connoting “vitality” and “energy” are strictly forbidden.
Impact on vapors:
Under new TPD laws, all e-liquid and e-juices are required to undergo costly emissions testing.
Emissions’ testing ensures that consumers are purchasing high-quality products that have undergone proper testing measures.
The aforementioned measures were implemented to assure consumers that their e-liquids are safe and have been laboratory tested and put through a rigorous standards-based production.
E-liquid flavors must be composed of safe ingredients proven to not pose harm to consumer health.
To meet TPD compliance rules, each ingredient must undergo emissions and toxicology testing.
This expensive process could prove to raise the prices of e-cigarettes liquids. Many vape e-juice and e-liquid suppliers may go out of business resulting from an inability to afford the expense involved for the uncompromising TPD compliance procedures.
Many vape e-juice and e-liquid suppliers may go out of business resulting from an inability to afford the expense involved for the uncompromising TPD compliance procedures.
TPD-compliant e-liquid will now show labeled batch numbers, ingredients, and quantity of each included ingredient.
E-liquid/E-juice – Maximum Nicotine Strengths
New TPD regulation:
Nicotine strengths of E-liquids shall not exceed 20mg/ml or 2%. TPD regulations intend that e-liquid nicotine levels will not be high enough to pose any significant possibility of danger to consumers.
Impact on vapors:
Smokers looking to quit cigarettes by switching to vaping may be deterred by the newly imposed lowered nicotine rules.
Studies show that roughly 200,000 UK vapors prefer higher levels of nicotine; new TPD maximum nicotine strength laws will undoubtedly encumber these users.
TPD Medicinal Requirements
New TPD regulation:
E-cigarettes with nicotine concentrations above 20mg/ml will be required to be licensed as medicine and thus subject to the same rules governing the sale of over-the-counter drugs.
TPD does not cover products containing nicotine that fall under the category of medicine.
Any product making the medicinal claim that it can help users quit or reduce smoking is subject to medicinal product regulation with a required MA approval.
Impact on vapors:
TPD-regulated e-cigarettes available on the market differ from those made with therapeutic claims and those containing over 20mg/ml of nicotine, which are regulated by the pharmaceutical legal parameters outlined in Directive 2001/83/EC of the Medicine Code.
E-cigarettes that contain less than 20mg/ml of nicotine fall under TPD regulation and are not considered having medicinal or therapeutic value.
Punishment and Additional Regulations
New TPD regulations:
Punishment for non-compliance could result in imprisonment for up to two years and unlimited fines.
Offenders are guilty of offences if they breach the following TPD provisions: labeling, emissions, additives, advertising, reporting compliance obligations, failure to submit necessary information to the Secretary of State
Additional regulations:
The advertising or promotion of electronic cigarettes and e-liquids is forbidden on media platforms including television, newspapers, radio, and magazines.
Impact on vapors:
Producers and sellers must follow strict regulation or face severe punishment. This may very well prove to impact the vape industry with limited product choices and higher price points.
E-liquid/E-juice – Maximum Volume
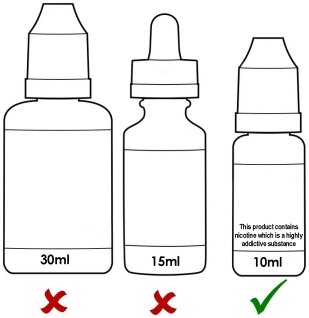
New TPD regulation:
Single refill containers are capped at a maximum 10 ml.Currently, E-liquids and juices come in 10ml, 20 ml, and 30ml sizing options.
Impact on vapors:
With new TPD regulations, vapors will be necessitated to purchase multiple dedicated bottles in order to obtain their accustomed hits.
MHRS Authorization Requirements
New TPD regulation:
The Medicines and Healthcare products Regulatory Agency has mandated that any new vape products must be notified to the agency and subsequently approved before allowed registration and entry on to the vape market.
Some products may be able to bypass the 6-month notification period if the MHRA approves their early submission to the agency.
In accordance with this regulation, producers who supply or intend to supply e-cigarettes and/or refill containers must register with the Secretary of State.
Also required to register with the Secretary of State are the following:
- UK-established retailers who currently in engage in or intend to engage in cross-bordered sales.
- Retailers outside the UK currently engaging in or intending to engage in cross-bordered sales of
- e-cigarette products with consumers residing in the UK.
Companies which notified the MHRA about their older, pre-existing stock were allowed a 6-month moratorium to sell their remaining stock until the TPD-imposed deadline of May 20, 2017.
Impact on vapors:
Products affected by MHRS/TPD regulations include all e-cigarettes, e-liquids, and e-juices.
The resultant cumulative effect on these products will mean a slowing down on vape-product innovation.
Consumers should expect a lack of new suppliers coming on to the vape market.
E-cigarette Product Packaging and Safety
New TPD regulation:
Designed to be safer for children, new TPD laws expressly state that vape containers must be durable enough to prevent breakage and accidental access and conditionally be “child-resistant” and “tamper-resistant.”
In complying with TPD mandates, e-cigarettes and e-liquids will now mostly be packaged within boxes clearly labelled with appropriate warnings.
Given the extensive warning information required, boxes will be utilized to give producers enough room to state the necessary warnings.
Impact on vapors:
The extra packaging required to accommodate the required TPD warnings has resulted in suppliers repackaging all of their e-liquid bottles.
This move has culminated in localized supply shortage and will prove to eventually have a high-impact on the environment.
New TPD Labeling Requirements for E-cigarettes
New TPD regulation:
All e-cigarette products must heed compulsory labeling requirements including:
- A health warning covering at least 30% of the unit package’s surface as well as any exterior packaging.
- Clearly displayed instructions for use.
- E-cigarette packaging must contain enclosed information leaflets. Packaging and included leaflet must display notifications on toxicity and addictive nature.
- Labeling must be clear and present a concise definition of the contents of the vape liquid/juice.
- Certain unapproved promotional descriptors with misleading information are expressly banned.
- E-liquid boxes must include warning labels on the front and rear of the box as well as on the enclosed bottle’s label. The box or label must not be edited or altered in any way and must state that the product contains the highly addictive substance of nicotine. Products containing zero-levels of nicotine are exempted from this rule.
- Bottles and labels must include clearly indicated warning and age restriction requirements. E-cigarettes containing zero-levels of nicotine are exempted from this rule.
- Boxes and labels must also include the product brand name, liquid name, per-dose nicotine content, address details and contact information, along with room for 5MM artwork and product bar code.
- Boxes or labels cannot reference or refer to tastes, smells, or other additives aside from flavorings.
- Packaging cannot resemble food products or cosmetic items.
- Packaging is forbidden to have any suggestive qualities insinuating that the e-cigarette product has health and lifestyle benefits, vitalizing, energizing, rejuvenating, healing, organic, or natural properties.
- E-cigarette products are not allowed to suggest that the product is less harmful than other products.
Impact on vapors:
While informative, the new labeling requirements may prove to lessen variety in the vape market, in addition to presenting vape producers with cumbersome costs labeling and packaging costs that might be financially unsustainable.
HOW DO I KNOW IF MY E-LIQUID IS TPD COMPLIANT?
TPD Compliant E-Liquid Is:
- 10ml or smaller
- Packaged in a box
- Comes with warning leaflets.
- Nozzles must be at least 1 cm long and emit no more than 20 drops per minute
- All e-liquids must be registered on the MHRA website and have an ECID number (European Community Identification Number)
- Comes with a clearly indicated warning stating: This product contains nicotine which is a highly addictive substance.
CONCLUSION
It shouldn’t come as too much of a surprise to see new regulations by the TPD on vaping products.
What may shock you, however, is the kind of regulations being put in place.
Forcing businesses to label their products as containing nicotine when there is clearly no nicotine included in the packaged item isn’t representing what the product actually is and may confuse some people.
What is also interesting is that e-liquid that contains nicotine is no longer allowed to be sold in bottles larger than 10ml, yet tobacco must be sold in a pack no less than 30 grams.
Where’s the logic here?
The fact is, there is none.
Having said this, there are many ‘hacks’ around these new laws, which we’ll be discussing in another article.
What do you think about the new TPD regulations? Are they fair?

